Search

ORIGINS is the largest study of its kind in Australia, following 10,000 children, from their time in the womb, over a decade to improve child and adult health.

News & Events
ORIGINS begins collecting baby teeth to unlock new insights into child health research.All participating families to receive $10 e-voucher for this generous donation.*

News & Events
International Women's Day 2025Hear from Avril Bezant, ORIGINS Data Coordinator, and Alexis Harun, ORIGINS Paediatric Coordinator, and be reminded that it’s never too late to pursue your passion along with their hopes for women entering a STEM career.

News & Events
ORIGINS Features at The CAHS SymposiumORIGINS featured heavily at this years Child and Adolescent Health Services (CAHS) Symposium, with presentations from our Co-Director, Professor Desiree Silva, and ORIGINS Data Manager, Dr Sarah Whalan.

News & Events
The Power of Longitudinal Cohorts – The 2024 ORIGINS ShowcaseYesterday, we were delighted to host close to 200 people at The ORIGINS 2024 Showcase, which explored the power of longitudinal cohorts, now and in

News & Events
Child has COVID? A reminder to think ORIGINS!ORIGINS is on the lookout for our Long COVID Study. As we enter a new wave of COVID in the community, we would like to remind participants to 'think
News & Events
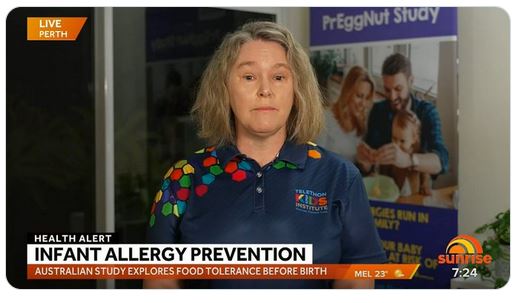
Changing advice for parents on food allergiesDr Debbie Palmer spoke to Ch7 Sunrise about the changing advice for parents about the development of food allergies in kids.

News & Events
Recruitment of active participants comes to an end for ORIGINSORIGINS has officially reached the study's five-year goal of recruiting 4,000 active participants to the project and has closed recruitment of new families into the active cohort.

News & Events
HBF and ORIGINS Helping Fathers to FlourishA new partnership with HBF will help fathers in their transition to parenthood

News & Events
Sharing the ORIGINS story on ABC DriveORIGINS chats to Geoff Hutchison about the impact of the study
