Search

News & Events
‘Mama’ deb’s dedication to saving children in Papua New GuineaChildren living in Papua New Guinea have good reason to call Clinical Associate Professor Deborah Lehmann ‘Mama Deb’.

News & Events
Sharing the power of data at TEDx PerthDr Hannah Moore was one of WA’s brightest minds chosen to speak at TEDX Perth in November last year, presenting her insights into the power of data in fighting infectious diseases to a sold-out crowd at the Perth Concert Hall.

News & Events
First week of school visits mark official launch of the SToP TrialThe The Kids Skin Health team has a busy six weeks ahead - visiting nine communities throughout the Kimberley region of WA as part of the first school surveillance activities for the SToP Trial.

News & Events
Ear health partnership brings brighter future for Aboriginal kidsA new partnership between The Kids Research Institute Australia, Dr George Sim and St John of God Murdoch Hospital will offer essential surgery at no cost for a group of Aboriginal children suffering severe ear infections.

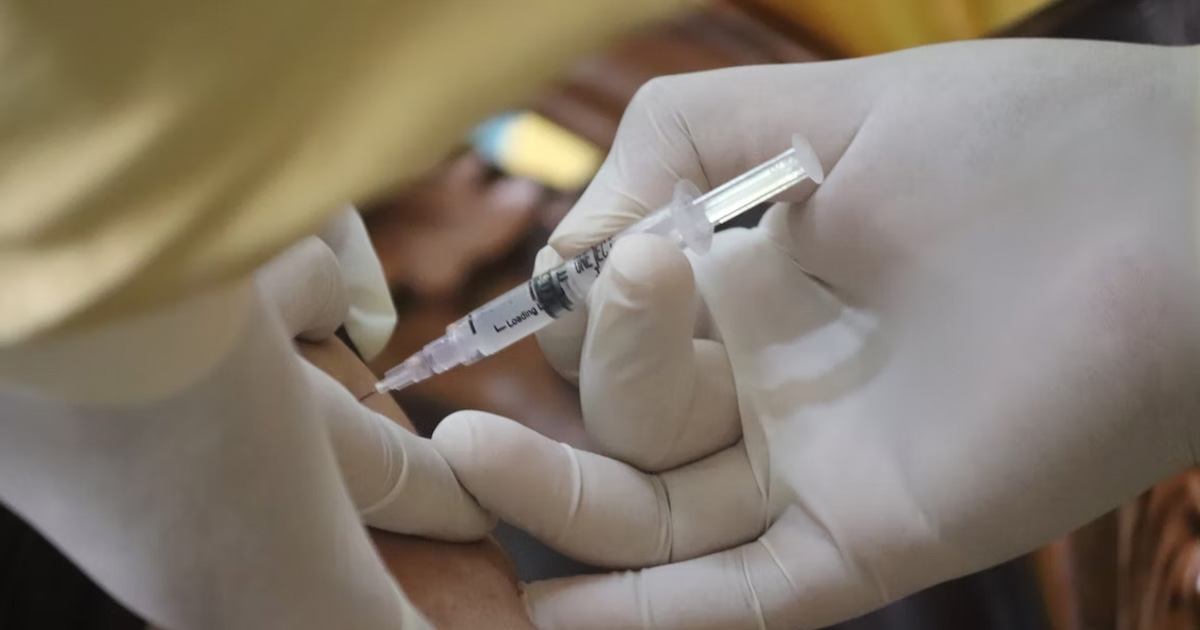
News & Events
Meningococcal vaccine provides extra protection for bubsIn 2017, a steep rise in cases of meningococcal disease caused by the W strain sparked a wave of concern for parents in Western Australia.

News & Events
The Kids researcher awarded Research Translation Projects grantA new research project aims to demonstrate how influenza vaccination in children could be a highly cost-effective health care intervention in Australia.
The Vaccine Trials Group was formed in March 1999 to provide a coordinated approach to the development, delivery, assessment and promotion of vaccines.

We are looking for healthy young adults to take part in a study that will help us learn more about a new pertussis vaccine that we hope will offer greater protection from whooping cough.
This is your chance to play a vital role in shaping Australia's COVID-19 vaccine program
Contact us If you'd like to get in touch, please contact us by phone or email. Phone: 0400 450 240 Email: rhyme@telethonkids.org.au Background The
