Search
Early-life antibiotic exposure is disproportionately high compared to the burden of culture-proven early-onset sepsis (CP-EOS). We assessed the contribution of culture-negative cases to the overall antibiotic exposure in the first postnatal week.
Late-onset sepsis (LOS) with Staphylococcus epidermidis is common in preterm infants, but the immunological mechanisms underlying heightened susceptibility are poorly understood. Our aim is to characterize the ontogeny of cytokine responses to live S. epidermidis in preterm infants with and without subsequent Gram-positive LOS.
The physicochemical compatibility of alprostadil injection with secondary intravenous (IV) drugs and 2-in-1 parenteral nutrition (PN) solutions used in Neonatal Intensive Care Unit settings was investigated.
Antibiotic exposure in neonatal intensive care units (NICU) is high. This study describes antibiotic use in very preterm infants and examines the association between duration of exposure and outcomes in blood culture negative (CN) infants.
The abundant skin commensal, Staphylococcus epidermidis, is the leading cause of late-onset sepsis (LOS) in preterm infants but rarely causes infections in term infants and adults. Staphylococcal virulence mechanisms and the role of the preterm immune responses in driving these life-threatening infections remain poorly understood.
The neonatal skin is central to early survival and immune development. Far from being a passive mechanical barrier, it integrates physical, chemical, and microbial defences that together protect the infant in the immediate postnatal period. In preterm infants, structural immaturity, reduced antimicrobial capacity, and altered microbial colonisation confer heightened vulnerability to infection and inflammation.
Impaired oxygen delivery or blood flow to the brain around the time of birth can cause injury. Hypoxic ischaemic encephalopathy is a leading cause of death and disability in term and near-term infants.
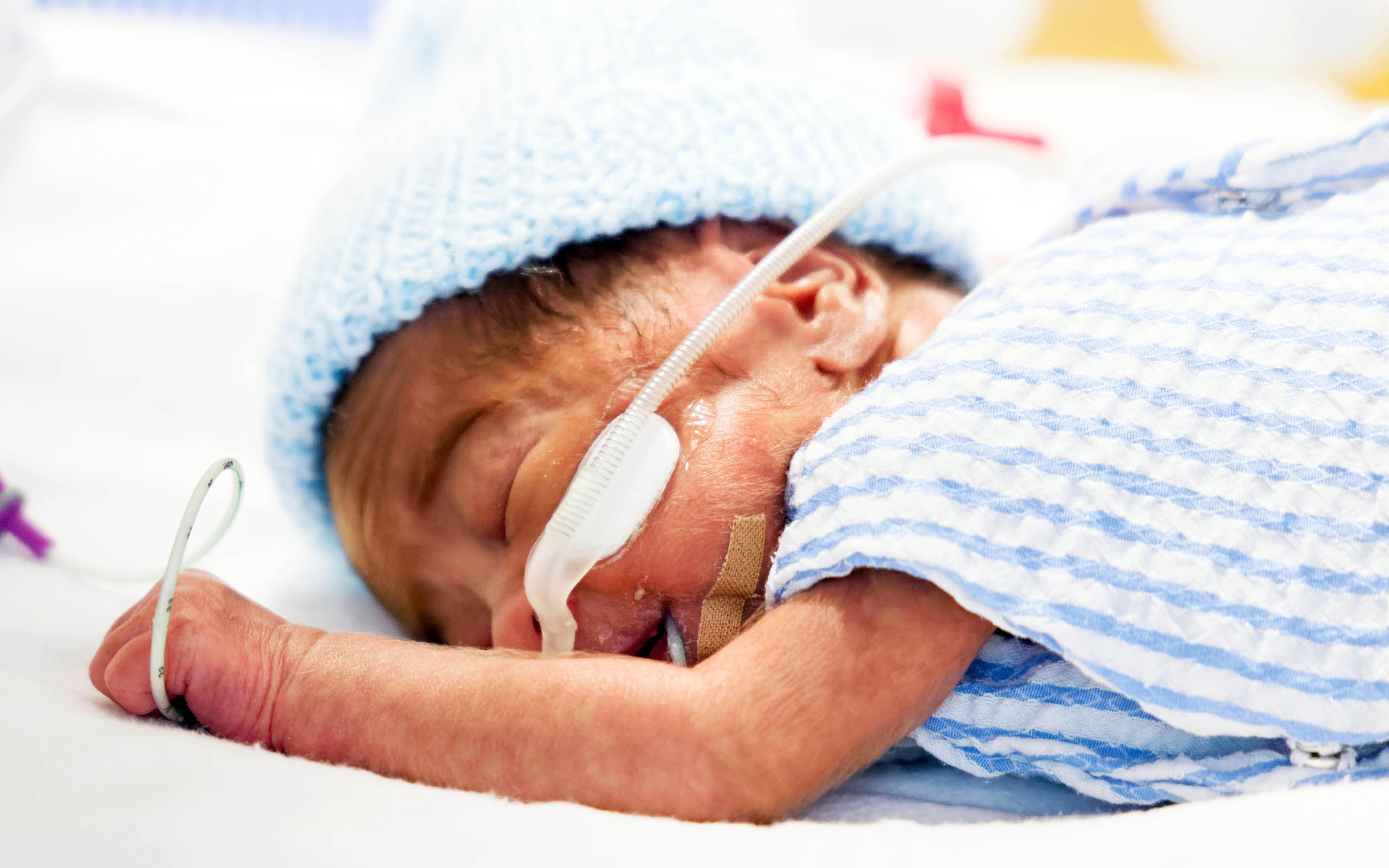
Preterm babies have a heightened risk of infection as their immune system is not mature. The Neonatal Health Team is exploring new ways to diagnose, prevent and treat infections in WA's smallest patients .
Delayed cord clamping (DCC) is an evidence-based intervention that reduces mortality, anaemia and disability in infants born <37 weeks' gestation who do not require immediate resuscitation. However, it is neither reliably recorded nor routinely implemented in Australia. The Wait a Minute or More study aims to reduce this gap between the evidence and practice by integrating timely sharing of cord clamping data with Evidence-based Practice for Improving Quality methods to increase the proportion of preterm infants receiving DCC for 60s or longer (DCC60).
Blood responses in very preterm infants with LOS are characterised by altered host immune responses that appear to reflect unbalanced immuno-metabolic homeostasis
